Reaction Mechanism and Kinetics for Ammonia Synthesis on the Fe(111) Surface | Journal of the American Chemical Society

Question Video: Calculating the Equilibrium Constant for Concentration Given the Initial Amount of Each Reactant | Nagwa

Calculate the equilibrium constant (Kc) for the formation of NH3 in the following reaction: N2 (g) + 3H2 (g) 2NH3 (g) At equilibrium, the concentration of NH3, H2 and N2 are 1.2 ×
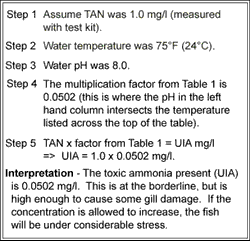
How to calculate your Un-ionized ammonia levels Information below collected from: Francis-Floyd, Ruth, Craig Watson, Denise Pett

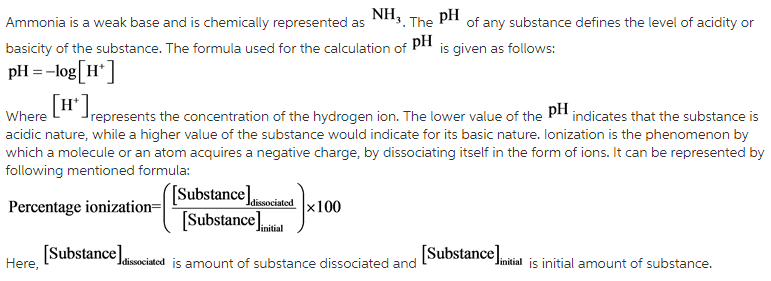
Calculate the pH of a 0.10 M ammonia solution . Calculate the pH after 50.0 mL of this solution is treated with 25.0 mL of 0.10 M HCl. The dissociation constant of ammonia, Kb=1.77xx10^(-5)

Step-by-step guide to calculating unionized (toxic) ammonia. UIA of... | Download Scientific Diagram

Step-by-step guide to calculating unionized (toxic) ammonia. UIA of... | Download Scientific Diagram

Ammonia Synthesis Production & Reaction | How Is Ammonia Made? - Video & Lesson Transcript | Study.com

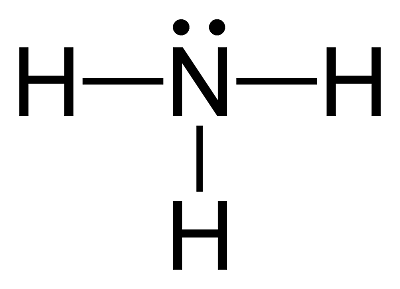




![Ammonia [NH3] Molecular Weight Calculation - Laboratory Notes Ammonia [NH3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonia-molecular-weight-calculation-300x150.jpg)