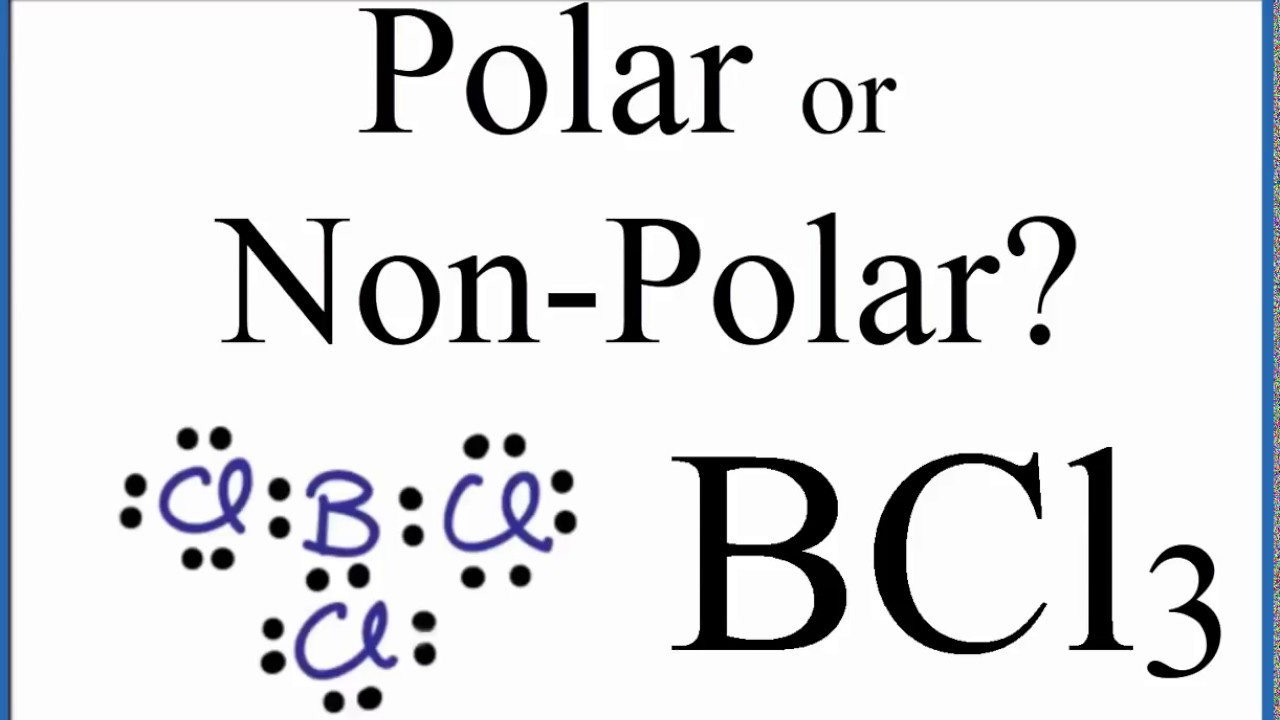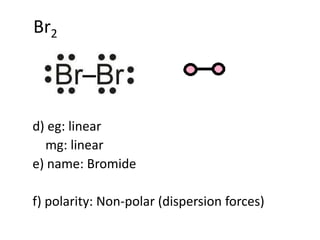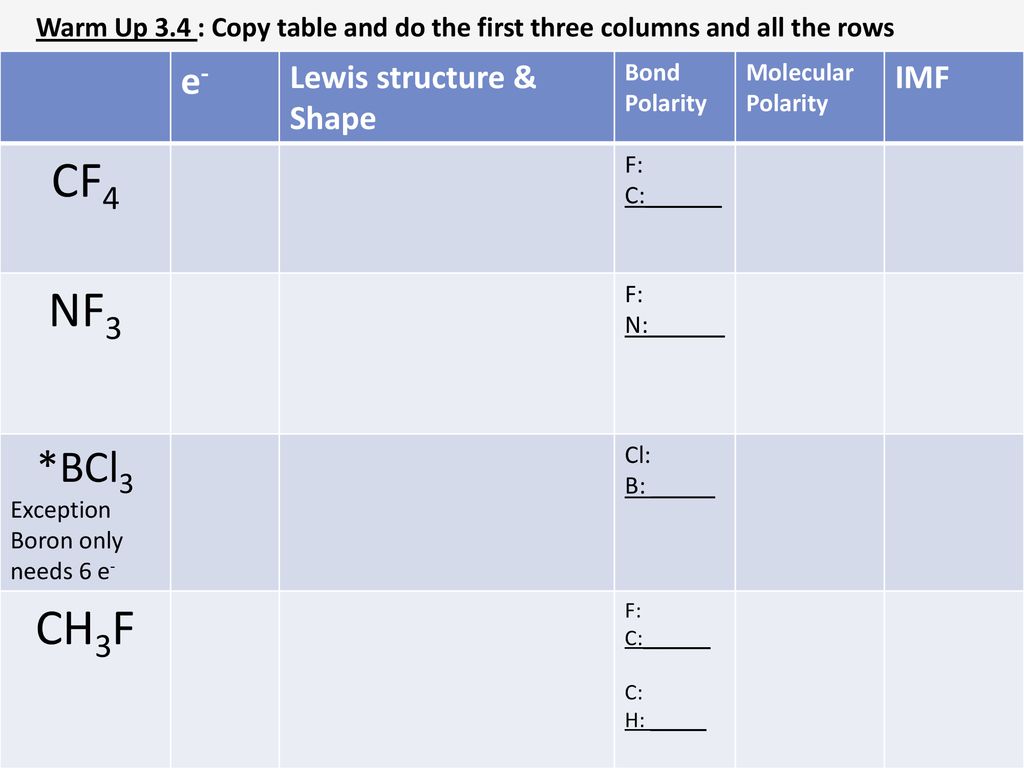
Polarization of an ionic bond means the distortion of the electron cloud of an anion towards a cation Polarization of an ionic bond results in an ionic. - ppt download

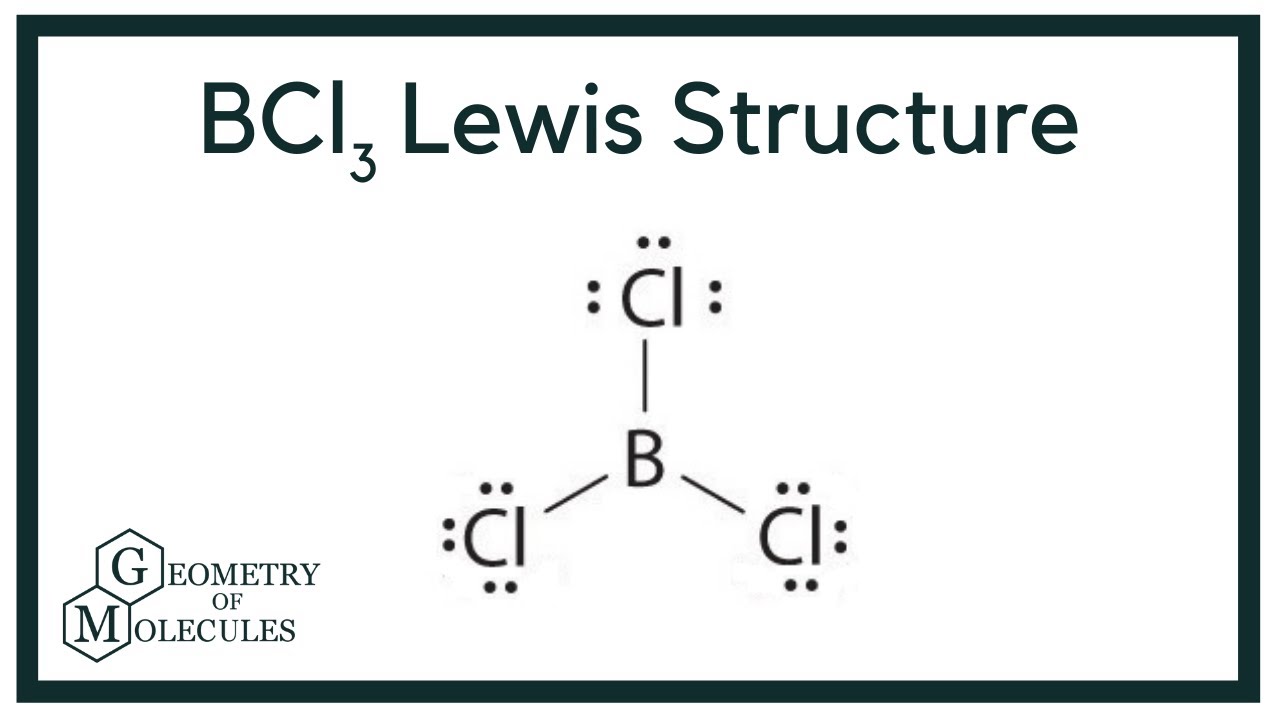
What do you expect for the B-Cl bond length in boron trichloride, BCl_3, on the basis of covalent radii? | Homework.Study.com