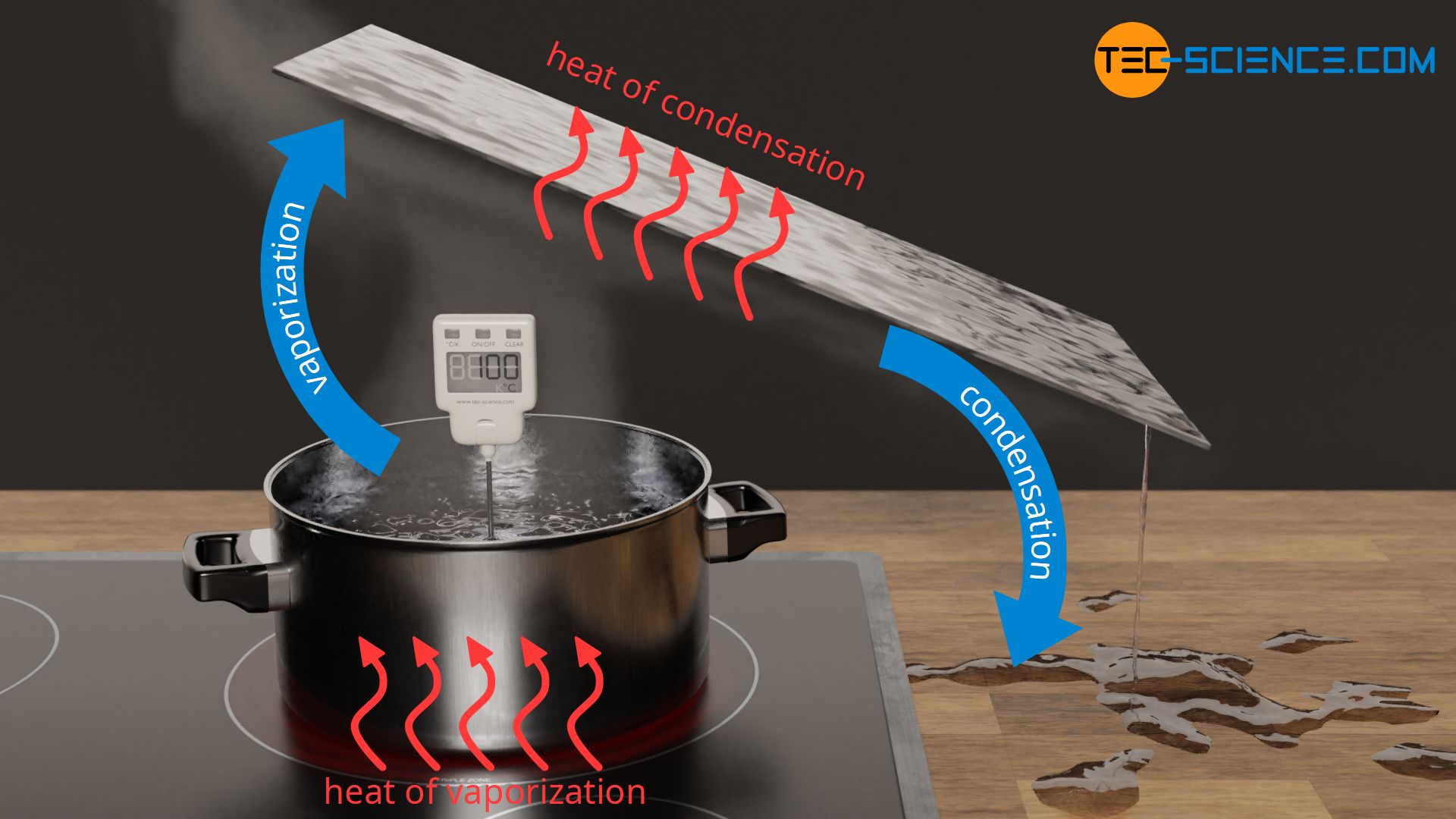
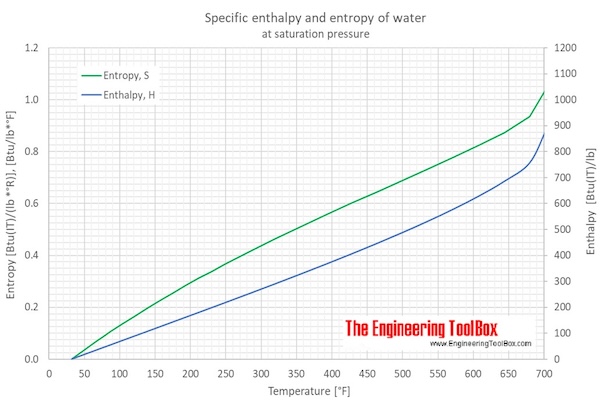
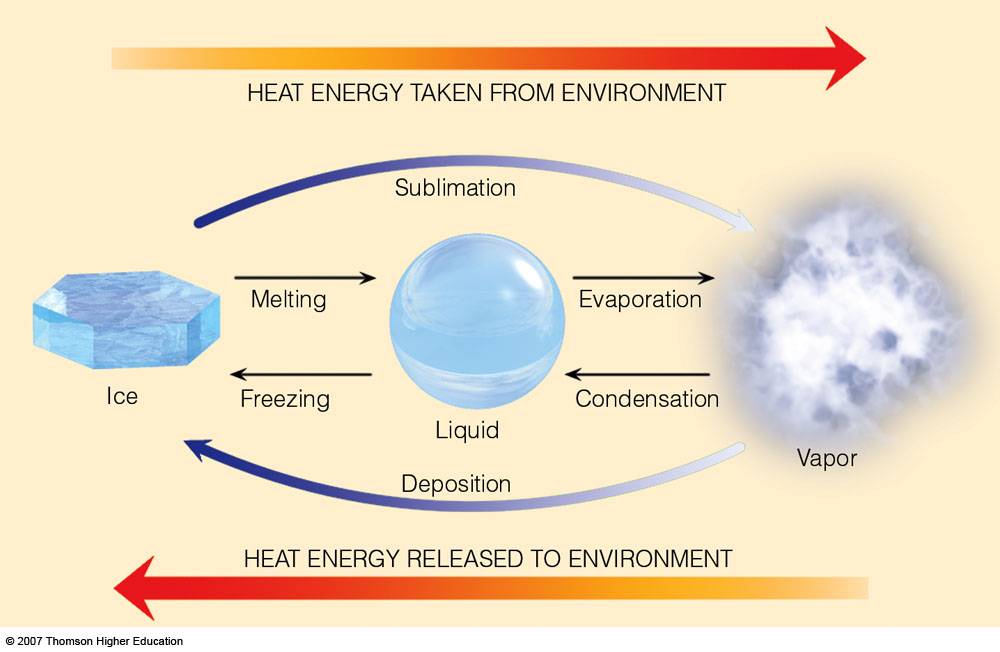
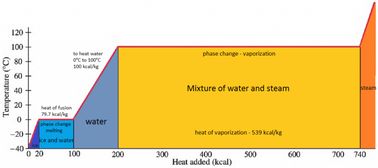
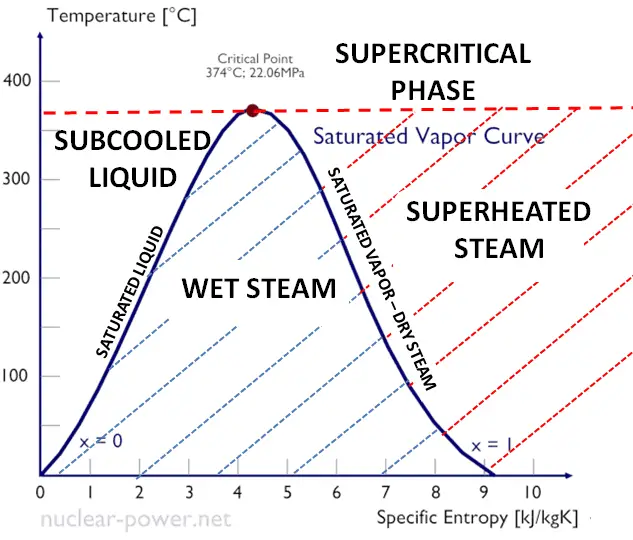
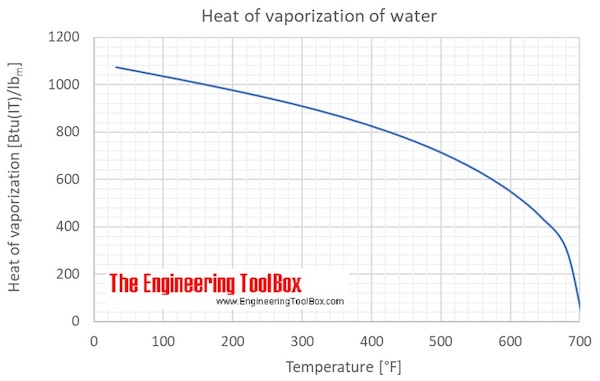
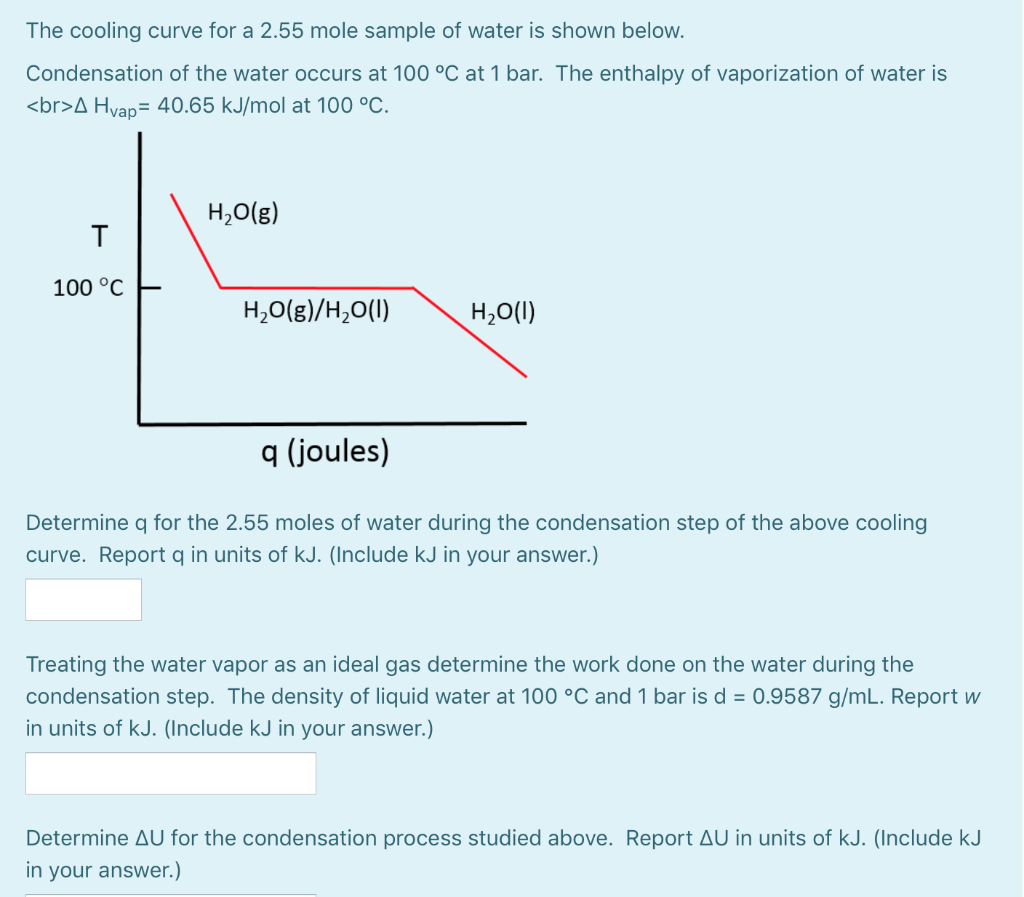
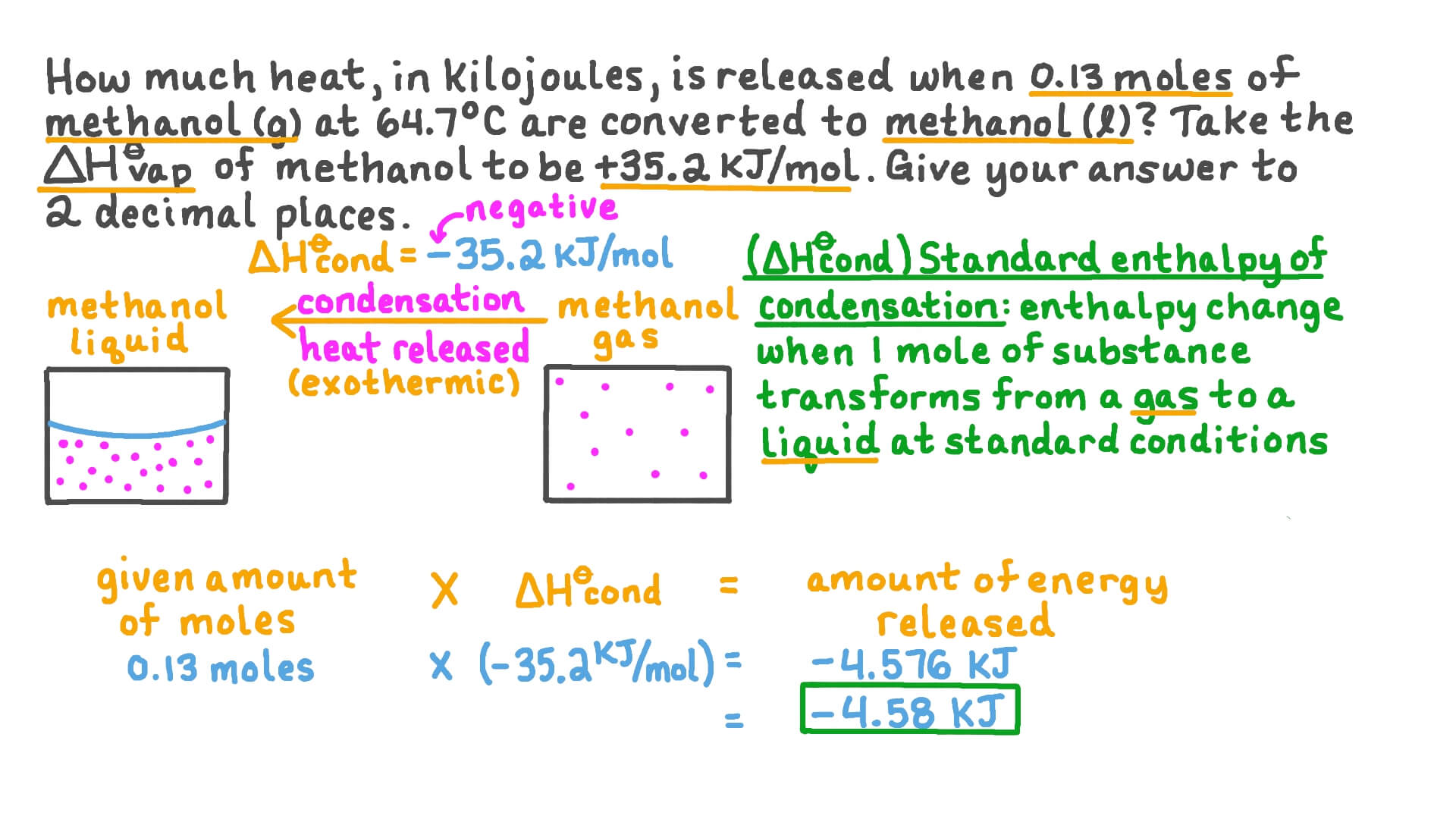
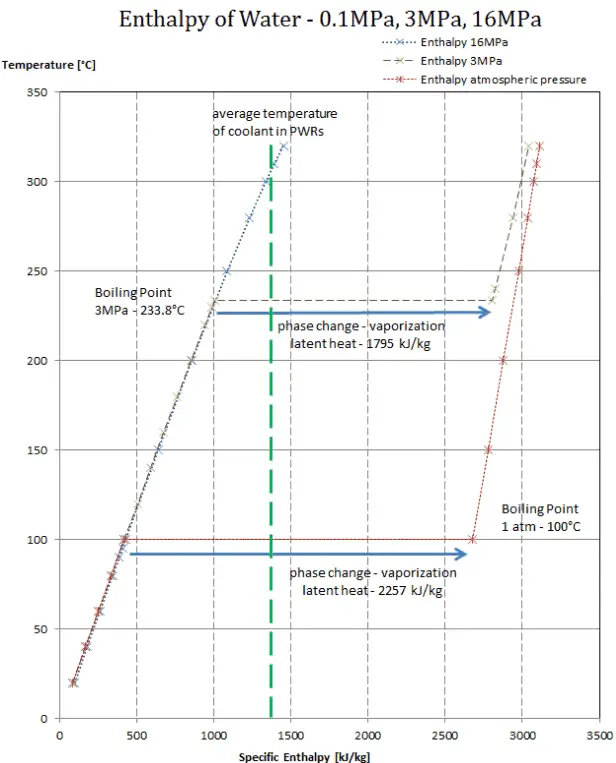
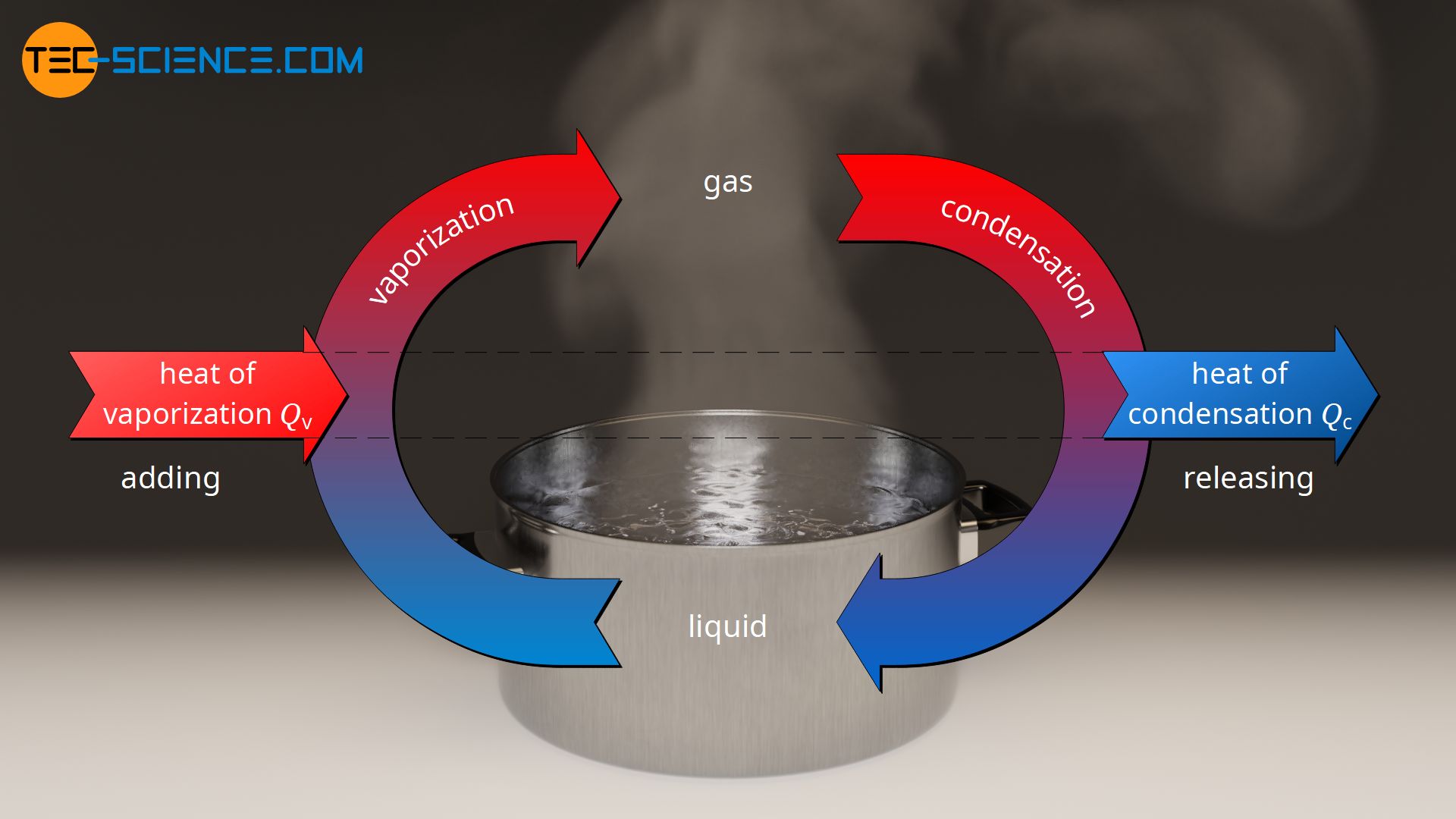
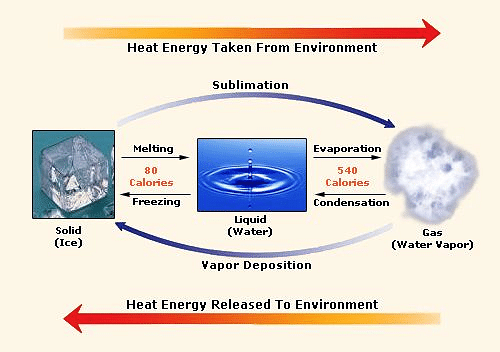
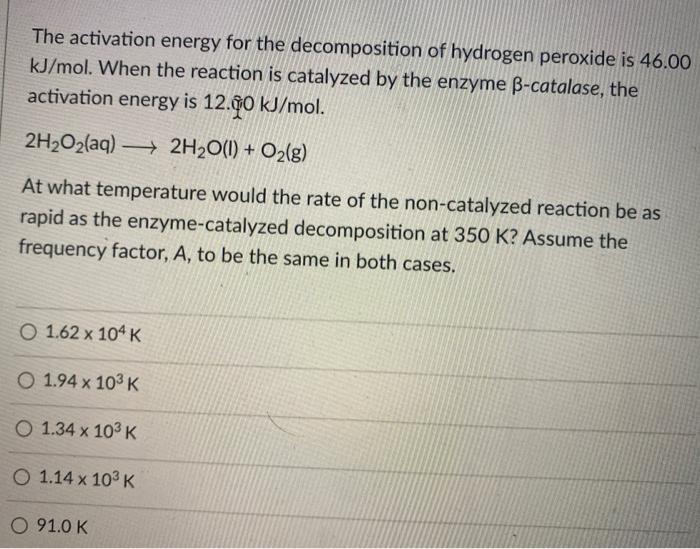
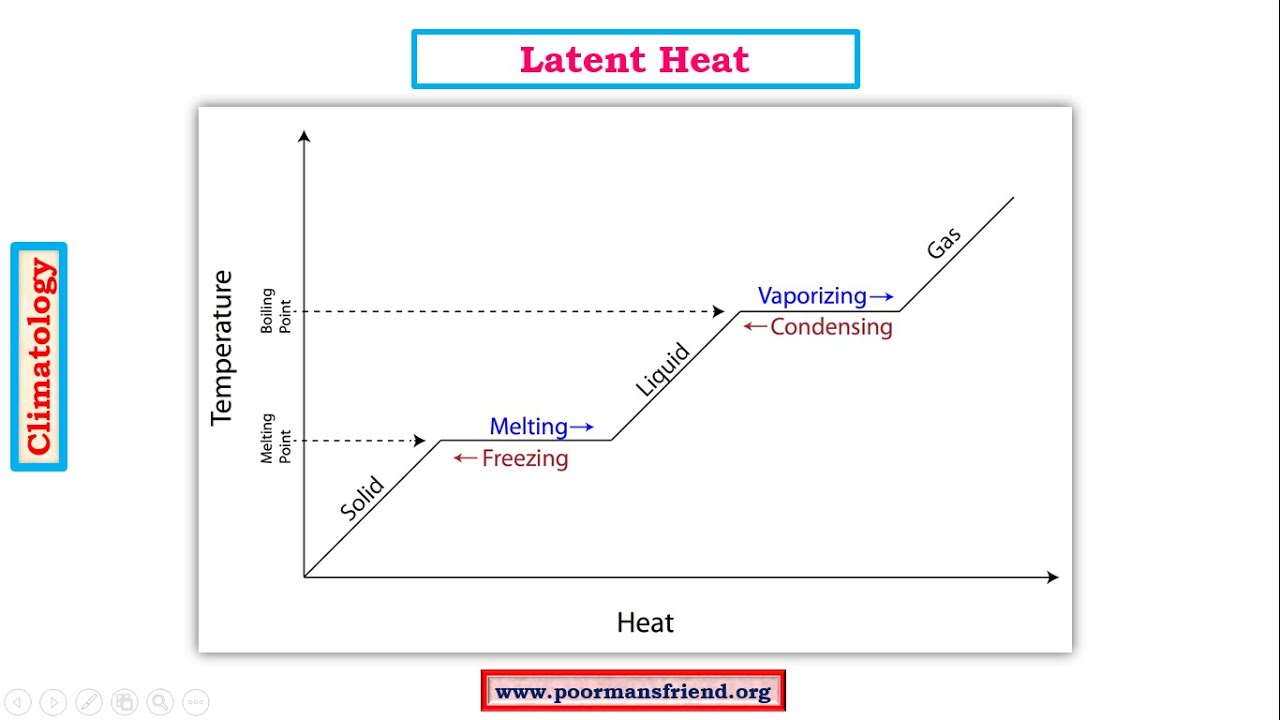
What is the difference between latent heat of vapourisation and latent heat of condensation? - Quora

Do Now 2NaHCO kJ Na 2 CO 3 + H 2 O + CO 2 Is this an endothermic or exothermic reaction? Calculate the amount of heat transferred when 36 g of. - ppt download
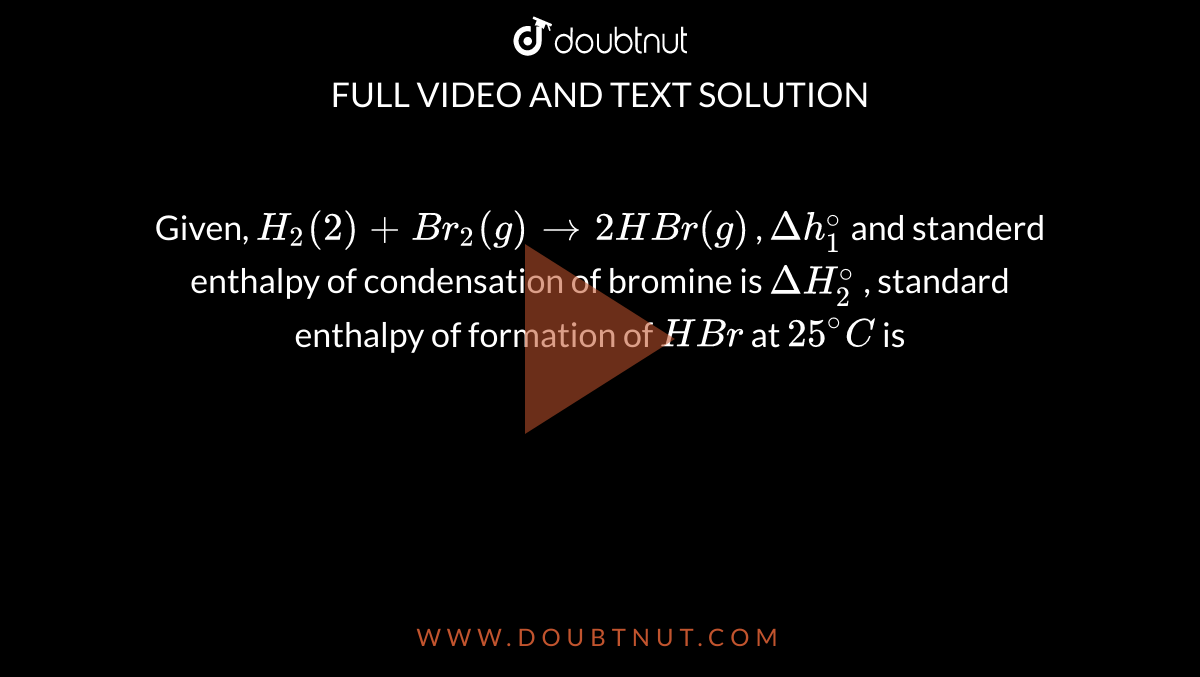
Given, H(2)(2)+Br(2)(g)rarr2HBr(g) , Deltah(1)^(@) and standerd enthalpy of condensation of bromine is DeltaH(2)^(@) , standard enthalpy of formation of HBr at 25^(@)C is

SOLVED: 'Calculate the molar enthalpy of condensation (AHccnd) for ammonia (NHe) when 50.0g of NHa gas turn into liquid at its boiling point; 68,500J of energy are released in the process'

Assuming the enthalpy of vaporization is 10 kJ/mol and the gas's molar volume at the onset of condensation is 1 L/mol, what is the enthalpy change for the C->B process is (in